Would you wear dirty eyeglasses or sunglasses? Just like these, microscope optics work better when they are free of dust and smears. Grime on your optics reduce the resolution, create artifacts, and degrade the image. In a worst-case scenario, your microscope could be rendered useless. Here are some basic guidelines for cleaning microscope optics. These steps increase in aggressiveness of the cleaning method so, for the best results, always start from the top and work your way down. It will be much easier to inspect and clean the optics (e.g. eyepieces and objectives) if you remove them from the microscope. You can also use an eyepiece as a handy 10x magnifier – just remove from the microscope and look through it from the back end about 1 cm from the item you are inspecting (see Figure 2b). With a little practice, you’ll be able to see smears, dust and even damage to the lens. Now let's review the cleaning process.
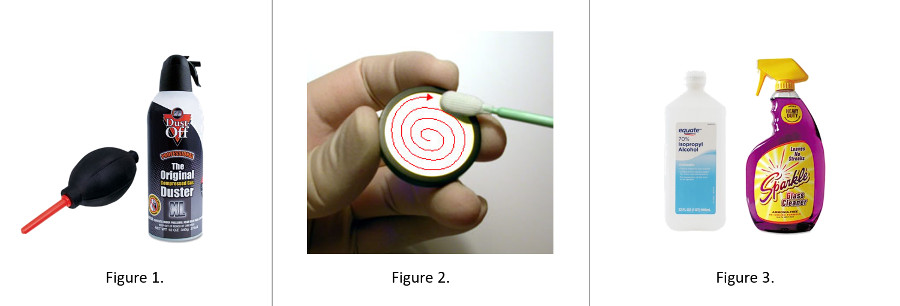
- Use a puffer or compressed air (Fig. 1)
Blow off surface dust by blowing air across it. A puffer or can of compressed air are ideal for this. This step also avoids any physical contact with the optical surface, minimizing the potential for scratching or smearing it.
- Use lens tissue
Many people initially reach for Kimwipes or facial tissue. Both are abrasive to optics, and facial tissue may also contain “softeners” which will add smears to the glass. Always use lens tissue. It is lint-free (no added dust, and non-abrasive). Here’s a trick, too. Wrap the lens tissue around a cotton-tipped applicator stick or Q-tip. You can also fold the lens tissue into a point. Don’t rub the glass, rather gently wipe the glass surface in a circular pattern, starting in the center and spiraling outward (see Fig. 2). Inspect the glass surface with your handy 10x magnifier and repeat the process if needed.
- Use water or warm breath
Just like breathing on your eyeglasses or a windowpane to make it fog up, you can add a warm breath to Step 2 to aid in removal of dust, debris, and some smears. Wetting the lens tissue with a little bit of water may also help. Always wipe the glass using the same spiraling method.
- Use alcohol or lens cleaner (WEAR NITRILE GLOVES)
After introducing lens tissue for some mechanical assistance in cleaning, the cleaning steps only increase in the aggressiveness of the solvent. When water/breath doesn’t work, the use of alcohol or lens cleaning solution (Fig. 3) is the next solvent to try. Sparkle glass cleaner has been a favorite, but dilute it 1:1 with distilled or deionized water (a.k.a. Milli-Q water). You can also use isopropanol or ethanol diluted to 70% in distilled or deionized water. Avoid getting these solvents on the rubber eyeshields of eyepieces. As discussed before, wrap lens tissue over applicator stick and dampen it (not soak) with the solvent before wiping. Use the spiral motion as before. Inspect and repeat as necessary.
- Use stronger solvents (WEAR NITRILE GLOVES), or seek professional help
Sometimes the stuck-on grime is more stubborn, such as dried-on immersion oil. The next step up in solvents would be xylol or a 1:1 mixture of ether-ethanol.
IMPORTANT! DO NOT SOAK THE OBJECTIVE IN THE SOLVENT as this may soften the cement securing the lens elements. You could use other organic solvents to remove even more stubborn crud (Residual Oil Remover, pure petroleum ether, etc.), follow same procedure as in Step 4, and use sparingly! Before even trying xylol or ethyl-ether, it may be safer and more effective to send your objective to a professional for cleaning.
Thanks for reading!